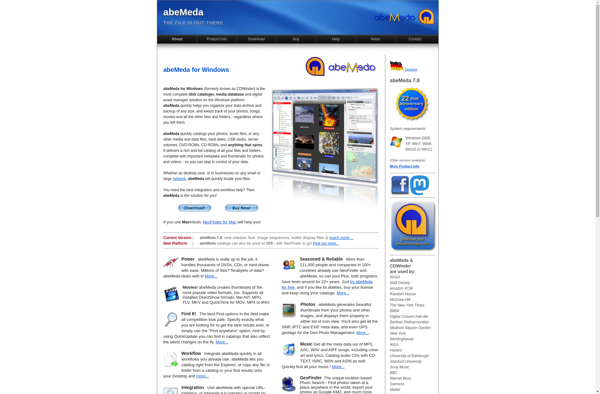
Description: abeMeda is an open-source electronic health records (EHR) system designed for small to medium-sized medical practices and clinics. It offers features like patient scheduling, billing, reporting, and more to help manage day-to-day administrative tasks.
Type: Open Source Test Automation Framework
Founded: 2011
Primary Use: Mobile app testing automation
Supported Platforms: iOS, Android, Windows
Description: Visual CD is a continuous delivery and release automation tool that helps software teams automate and standardize release pipelines. It provides built-in integrations with popular dev tools, configurable deployment workflows, rollbacks, and release reporting.
Type: Cloud-based Test Automation Platform
Founded: 2015
Primary Use: Web, mobile, and API testing
Supported Platforms: Web, iOS, Android, API