Periodic Table of Elements
Periodic Table of Elements: Comprehensive Reference Guide
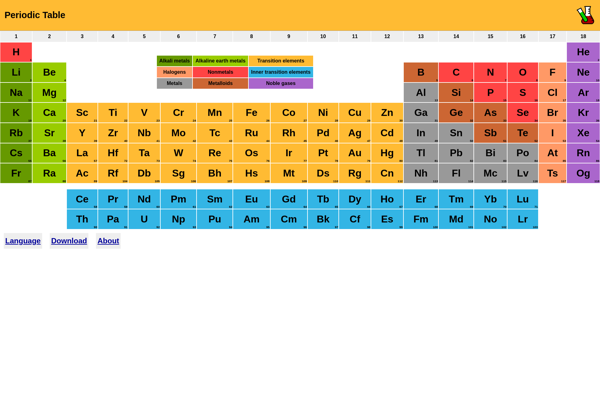
The Periodic Table of Elements is a tabular display of the chemical elements, arranged by atomic number, electron configuration, and recurring chemical properties. It provides a quick reference for the properties of each element.
What is Periodic Table of Elements?
The Periodic Table of Elements is a tabular arrangement of the chemical elements, ordered by their atomic number, electron configuration, and recurring chemical properties. The table provides a useful framework for analyzing chemical behavior and predicting the properties of elements based on periodic trends.
The standard layout of the table consists of rows (periods) and columns (groups). Elements with similar properties are placed in vertical groups. Horizontal rows arrange elements according to increasing atomic number and highlights periodic trends, such as ionization energy, electron affinity, and electronegativity, allowing predictions about chemical properties to be made. Metals, nonmetals, and metalloids are also grouped together.
The origins of the periodic table date back to the early 19th century, but it was Dmitri Mendeleev who first published the influential early version in 1869. Advances in quantum mechanics and physics in the 20th century solidified the basis for electron configurations that underlie the table. Updates and expansions to the table continue as new elements are discovered.
The periodic table is widely used as an essential tool by students beginning their study of chemistry, allowing them to visualize recurring trends and patterns among the elements. It also serves as an authoritative and universal reference for scientists, engineers, and technical professionals working across various chemistry-related fields.
Periodic Table of Elements Features
Features
- Displays all 118 elements with symbol, name, atomic number, atomic mass
- Interactive periodic table allows clicking on each element to see more details
- Includes group, period, and category information for each element
- Shows electron configuration, electronegativity, and other properties
- Allows searching and filtering of elements
- Includes interesting facts and figures about each element
Pricing
- Free
Pros
Cons
Official Links
Reviews & Ratings
Login to ReviewNo reviews yet
Be the first to share your experience with Periodic Table of Elements!
Login to ReviewThe Best Periodic Table of Elements Alternatives
Top Education & Reference and Science and other similar apps like Periodic Table of Elements
Gnome Chemistry Utils
Kalzium
Elements: The Periodic Table
Periodica - Periodic Table

ChemistryLab
Atomic - Periodic Table
Interactive Periodic Table in JavaScript
Periodic Table Classic
EniG. Periodic Table of the Elements
WebElements
GPeriodic